Story highlights
Scientists in England have crossed a major hurdle to creating blood cells in the lab
Using stem cells, the researchers made 'immortal' cells that create an unlimited supply of red cells
It will be years before these cells are put to use in the hospital, and only for a small subset of patients
Volunteer blood donors will still be needed
For decades, scientists have sought to create red blood cells in the lab – a “holy grail” that some hoped could ease regional blood shortages, especially for people with rare blood types.
But now British researchers say they have overcome a major barrier that has plagued many scientists: creating enough red cells to fill a blood bag. Their findings are published in the journal Nature Communications.
“When we kept (the cells) continually dividing for a year, we were quite excited,” said Jan Frayne, a biochemist at the University of Bristol and one of the study’s lead authors.
The latest study “is a dramatic step forward because it gives us the view that we can actually scale up to whole units of blood,” said Dr. Harvey Klein, chief of the NIH Clinical Center’s Department of Transfusion Medicine. Klein was not involved in the study.
Two to three drops of blood may contain a billion red cells, according to the American Red Cross.
“This technology gives us that particular dream, or at least it brings us a lot closer,” said Klein.
There will be blood
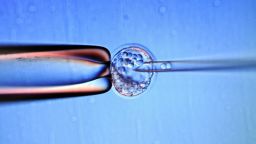
To ramp up production, the UK researchers infected stem cells with cervical cancer genes. By inserting cancer genes from human papilloma virus (HPV) into bone marrow cells, Frayne and her colleagues were able to create the first adult red blood cells that could multiply an infinite number of times. These cells are referred to as “immortal.”
The concept may be a familiar one to those who have read the book “The Immortal Life of Henrietta Lacks,” in which a related strain of HPV led to the production of HeLa cells, which are widely used in scientific research. These cells were taken from a cervical cancer biopsy from Lacks, who passed away in 1951 but whose cells still multiply in laboratories today.
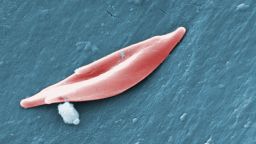
As the red blood cells mature, they spit out the nucleus – the core that houses their DNA – giving the cells a signature round, dimpled shape. Frayne and her colleagues filtered those cells from the rest, so the final batch did not contain the active cancer genes.
Frayne said that a small number of these stem cells can be found in a simple blood draw, too; there’s no need to do an invasive biopsy of the bone. Since her team completed the study last year, she said, they have already created two new immortal cell lines this way.
“It’s a brilliant approach, and they seemed to have solved several of the really important bottlenecks,” said Dr. Robert Lanza, Chief Scientific Officer at the Astellas Institute for Regenerative Medicine.
Lanza is no stranger to the research; he tried to solve the same problem years ago using embryonic stem cells.
But his cells didn’t eject the nucleus well enough, and fetal blood cells have too tightagrip on oxygen; they are less likely to drop off the oxygen where it needs to go. Eventually, though, he abandoned the research because “it’s not really commercially viable.”
First blood
Many others have attempted to create blood in the lab, using stem cells from umbilical cords and other sources. But these stem cells fizzle out and stop dividing at a certain point.
“It’s almost like they desperately want to carry on differentiating” into mature cells, Frayne said.
In 2011, a group of French researchers transfused lab-grown red blood cells – which grew from stem cells, though not Frayne’s endless supply – into one human. The cells functioned and survived normally.
Frayne said that the first human trials will begin in England later this year, though they will not be using the immortal cells from her new study. Making the new cells under industry standards, Frayne said, could take at least several more years.
A number ofother prior studies have sought to create oxygen-carrying liquids without the need for blood cells, but none of them have proved to be widely usable. In fact, a 2008 analysis found that they carried an increased risk of heart attack and death. A blood substitute called PolyHeme was famously rejected by the US Food and Drug Administration after 10 patients suffered heart attacks out of 81 who received it.
Whole blood contains a lot of other bits and pieces that may not necessarily be grown in a lab, said Lanza: blood-clotting platelets, proteins, immune cells and ions like iron.
But Lanza also said that the advantage of lab-grown blood is that it avoids common problems for patients who require multiple transfusions over their lifetime, such as those with sickle cell disease. For example, iron, which can be toxic at high concentrations, can accumulate with successive blood bags, which are given during a transfusion. Human blood, though rigorously tested, also carries a very small risk of transmittingdisease.
And stem cells could be used to create Type O cells, fit for nearly any patient’s IV, Lanza said. Known as the “universal donor,” Type O is the blood type most often requested by hospitals, but it is frequently inshort supply, he said.
But where Lanza really expects to see this technology is on the battlefield.
Red blood sells
The Department of Defense technology research agency, known as DARPA,has funded similar studies in the past, such as a “blood pharming” study with a medical device company formerly known as Arteriocyte.
Lanza, who met with DARPA officials about his own blood cell research in the past, said that the military wants to use lab-grown blood “for patients who have massive blood loss, particularly in the battlefield, where a soldier is blown up by a bomb and there isn’t time for blood typing.”
“I think the goal ultimately is to put this on the back of a Humvee,” he said.
That research, however, met the same obstacles other scientists facedin the past, Klein said.
“They were not able to make sufficient amounts blood at any kind of reasonable cost,” said Klein, who also serves on the FDA Blood Products Advisory Committee. Though familiar with the DARPA research, he was not involved in evaluating its products.
To mass produce blood in the lab, Frayne and her colleagues would need lots of expensive liquids to grow the cells and a battery of new equipment that complies with manufacturing standards – all of which will cost money.
“To make big huge vats of it would be outside of our ability in a research lab,” she said. “We’d have to have company interest.”
A hospital in the US might pay hundreds to thousands of dollars to purchase and test a unit of donated blood, and it may charge far more to transfuse it to patients. Producing a pint of blood using her method, Frayne said, would likely be several times more expensive than buying bags from blood donors in the UK.
But Frayne is optimistic that costs will come down. She hopes that lab-grown cells will be shown to last longer, and therefore doctors might need to use less blood less frequently. That’s because stem cells can be collected while they’re young, Frayne said, while human blood has cells of all different ages. Many donated blood cells die not long after transfusion.
Collected blood expires, too. Currently, the Red Cross, which claims to provide 40% of the country’s blood supply, stores red blood cells for up to 42 days.
That aside,Klein said that lowering the cost to $1,000 to $2,000 per unit of blood would make these cells worth the price for a small subset of patients who have rare blood types or need regular transfusions. For the typical hospital patient, however, it would probably not be very practical or cost-effective, he said.
But it is their willingness to invest money in the research, Klein said, that may have led to the British team’s success where the US and other countries have faltered.
“They have put a great deal of financial muscle behind doing this on a national basis, which we simply haven’t seen in the United States,” he said, adding that perhaps there was an element of “healthy skepticism (in the US) that maybe it will never in our lifetime be practical.”
“I don’t share that skepticism,” he said.
Bad blood?
But what about the rogue red cell that slips through the filter with its cancer genes still intact? Lanza calls these cells “escapees.”
“When you’re dealing with such huge numbers of cells,” said Lanza, “there may be a few of these cells that would slip in.”
Frayne said that these cells are highly unlikely to cause any form of blood cancer. The cancer genes are only switched on by a certain antibiotic, and by the time the cells are collected, any remaining nuclei are no longer working. Before blood transfusion, radiation can also be used to destroy any leftover DNA without affecting normal cells, she said.
Still, Frayne said, “These are all really good points to be raising, and they need to be looked at.”
But none of these concerns have slowed a deluge of requests to use her cells, Frayne said, though perhaps not from whom you’d expect. It’s not blood banks hoping to capitalize on a new, if untested, method. In fact, it’s other researchers who, until now, have not had an unlimited way to study diseases like malaria, which infect red blood cells. “That’s where all my requests are coming from,” she said.
Join the conversation
Klein, Lanza and Frayne all said lab-grown blood cells are not meant to replace blood donors. To fill a national blood service, or even a single hospital, will require another major leap in the research.
“They’re not going to put the Red Cross out of business,” said Lanza. “Volunteer blood donations are always going to be the first line of defense – but with this technology, you have a safety net.”