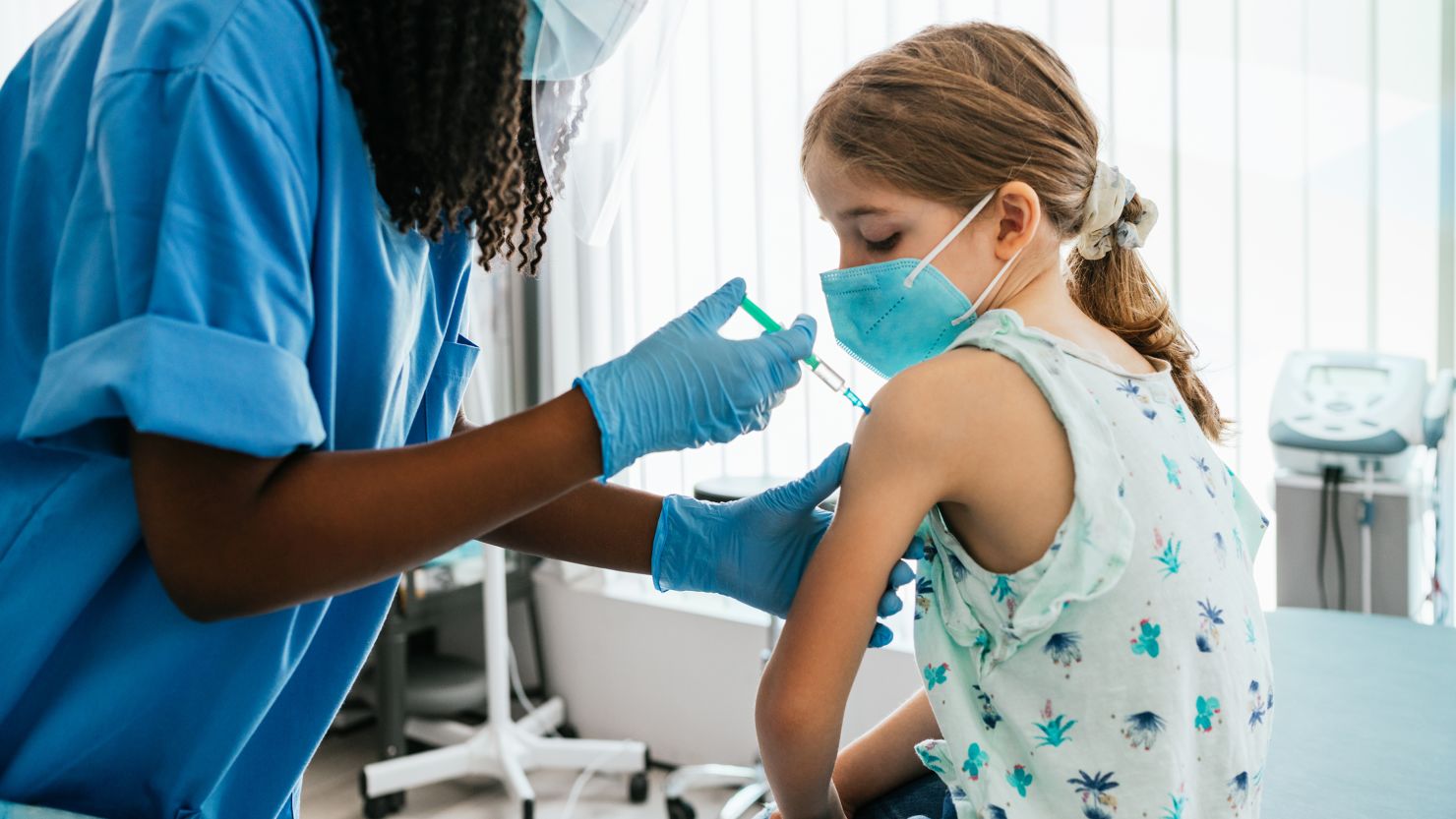
Everyone ages 6 months and older should get an updated Covid-19 vaccine, the US Centers for Disease Control and Prevention said Tuesday, to help lower the risk of severe illness, hospitalization or death from the coronavirus.
Dr. Mandy Cohen, director of the agency, signed off on recommendations from the Advisory Committee on Immunization Practices or ACIP, a panel of experts that advises the CDC on its vaccine recommendations.
The CDC said in a news release that the shots from Pfizer/BioNTech and Moderna will be available this week.
“Vaccination remains the?best protection?against COVID-19-related hospitalization and death,” the agency said. “Vaccination also reduces your chance of suffering the effects of Long COVID, which can develop during or following acute infection and last for an extended duration. If you have not received a COVID-19 vaccine in the past 2 months, get an updated COVID-19 vaccine??to protect yourself this fall and winter.”
The endorsement from the CDC and the committee means the vaccines will be covered by public and private insurance plans.
The new vaccines have been updated to fend off the currently circulating viruses that cause Covid-19.
They teach the immune system to recognize the spike proteins of the XBB.1.5 viruses, which are still circulating and have given rise to a crop of new variants that are now dominating Covid-19 transmission. The new shots contain a single strain of the virus, unlike last year’s shots, which contained two strains. Those older shots are no longer authorized for use in the US.
The updated vaccines are arriving amid a late summer uptick in Covid-19 hospitalizations and deaths.
The?most recent data?from the CDC shows that hospitalizations for Covid-19 rose 9% last week compared to the week prior. Despite the increase, hospitalizations are still roughly half of what they were during last winter’s peak.?Weekly Covid-19 deaths also climbed in August.
New data presented to the advisory committee Tuesday by Dr. Fiona Havers of the CDC’s National Center for Immunization and Respiratory Diseases showed that the highest rates of hospitalizations and deaths are in the very old and very young: adults over 75 and infants younger than 6 months. All other groups are at lower risk for severe outcomes.
Additionally, clinical trial data presented Tuesday on the effectiveness of the updated vaccines didn’t include children under 12, which left ACIP member Dr. Pablo Sanchez, a pediatrician at Nationwide Children’s Hospital in Ohio, feeling uncomfortable about making a blanket recommendation for everyone 6 months and older. He was the committee’s only no vote.
“I just want to be clear that I am not against this vaccine,” Sanchez said. “The limited data that are available does look great.
“We have extremely limited data on children … and I think that needs to be made available … to the parents,” he said in explaining his discomfort.
Other members felt that making more tailored risk-based recommendations, which would require that certain groups discuss Covid-19 with a health care provider before they could get a shot, would unnecessarily limit access to the updated vaccine.
“There is no group that clearly has no risk from Covid,” said Dr. Sandra Fryhofer, who was representing the American Medical Association at the meeting. “And even children and adults with no underlying conditions can still experience severe illness due to Covid.”
Fryhofer said that with immunity beginning to wane and new variants emerging, we’re all becoming more susceptible, and that this will probably increase over time.
“Today’s discussion makes me very confident that this new vaccine will help protect us from Covid, and I really encourage ACIP to vote for a universal recommendation for those 6 months and older,” she said in deliberations before the vote.
Clinical studies presented by Moderna, Pfizer and Novavax on Tuesday showed that all the updated shots significantly boosted antibodies against currently circulating coronavirus variants, suggesting that they will provide good protection against the predominant variants.
Two mRNA vaccines, from Pfizer and Moderna, were approved and authorized by the US Food and Drug Administration on Monday. A third updated vaccine, made by Novavax, is still under FDA review, so ACIP was unable to make specific recommendations for its use.
However, with the way it worded the vote, the committee agreed to recommend any authorized or approved XBB-containing vaccine, so it won’t need to meet again to consider that vaccine should the FDA clear it, as it is expected to do.
The committee said everyone ages 5 and older should get at least one dose of an updated mRNA vaccine against Covid-19 vaccine this year.
Children 6 months through 4 years of age, who may be getting their vaccines for the first time, should get two doses of a Moderna vaccine and three doses of a Pfizer Covid-19 vaccine, with at least one of the doses being an updated 2023 shot.
The committee also made recommendations for people who are moderately or severely immunocompromised. To be up-to-date, those with low immune function should have had at least three doses of Covid-19 vaccine, with at least one of those doses being an updated shot.?They also have the option to get an additional updated vaccine later in the year.
The committee has not decided whether seniors 65 and older might need another dose of an updated vaccine in a few months. Seniors became eligible for a second dose of a bivalent Covid-19 vaccine this past spring.
This is the first time Covid-19 vaccines will be made available through the commercial market. The manufacturers revealed the list prices of their vaccines Tuesday, with wholesale prices of $120 to $130 per dose.
Under the terms of the Affordable Care Act, many commercial insurance plans available through the government or an employer have to provide vaccines at no cost. So some people will still pay nothing out-of-pocket for their Covid-19 vaccines.
Get CNN Health's weekly newsletter
- Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
But an estimated 25 million to 30 million adults don’t have health insurance or don’t have enough insurance.?They will be eligible for free Covid-19 vaccines through the government’s Bridge Access Program, which will provide vaccines at health departments and federally qualified health clinics.?Bridge Access providers will be added to the?vaccines.gov?website this week, which will help people find locations to get the free vaccines.
Children from low-income families will be able to get free vaccines through the CDC’s Vaccines for Children program.